TMPRSS2, a longstanding PCF drug target, could be the key to why COVID-19 is entering the lungs. Instead of starting from zero like many other aspects of COVID-19 research, PCF’s historical investment is giving researchers a starting position that is much closer to the finish line.
Why is this important? Because time is of the essence. COVID-19 kills more than 3 people every minute. It is imperative that this work be done now, separate from and in parallel to, the work of developing a vaccine. While a vaccine may take another 12-18 months or more, treatments against TMPRSS2 could be effective for the sickest patients in as soon as 4 months.
This simple cartoon illustrates how we might use what we know to block COVID-19 from doing its devastating damage to the lungs.
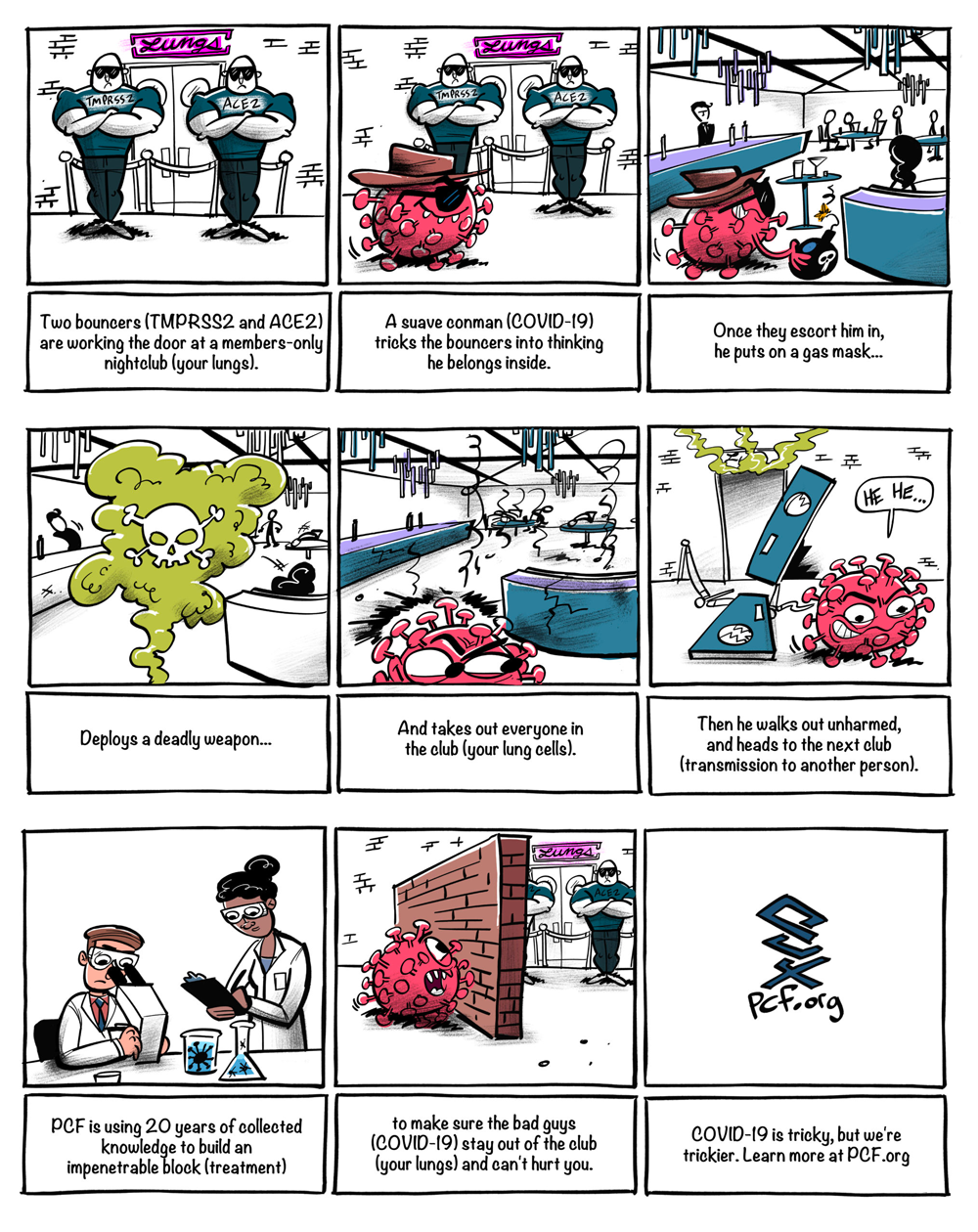
Put simply, TMPRSS2 is like a door handle that lets COVID-19 into your lungs. PCF is actively working on clinical trials for two different classes of anti-COVID-19 drugs: one will make the door handles fall off, and one will make the door handles impossible to turn. Either way, the outcome is the same: COVID-19 will no longer be able to get into the lungs and this will stop COVID-19 from turning deadly.
Nearly 2.3 million people have been infected with COVID-19 in the US and global deaths have surpassed 450,000. COVID-19 is a tricky opponent for many reasons: symptoms vary widely from person to person, people are often symptom-free for many days while shedding and spreading the virus, and a substantial number of infected people have mild or no symptoms at all. To complicate things further, it is unknown whether people who have recovered can be re-infected. Here’s one thing we do know: men are 2-3 times more likely to die of COVID-19 than women.
In the age of COVID-19, the Prostate Cancer Foundation has instantly redeployed its research enterprise to help solve this deadly problem. In collaboration with pulmonologists and infectious disease experts PCF intends to get to the bottom of the disparity between how COVID-19 affects men and women.
Over the last 20 years, PCF has funded millions of dollars of research into TMPRSS2 and prostate cancer; thanks to this foundational research, we know TMPRSS2 could potentially be a drug target for COVID-19. PCF’s Prostate Cancer Research Enterprise is applying our body of knowledge about TMPRSS2 to the solve the COVID-19 problem.
Thus far, we have invested over $5 million in the study of TMPRSS2. Without this foundational work, we wouldn’t know what we know today – and we wouldn’t be nearly as close to a solution. Prior to this, PCF had funded work on TMPRSS2 at UCLA, the University of Minnesota, and the University of Michigan. PCF lead investigators in TMPRSS2 R&D from 1999 to the present include: Arul Chinnaiyan, Peter Nelson, Leroy Hood, Matt Rettig, and Charles Ryan.
PCF has now organized and funded a multi-disciplinary research community to tackle this problem, including over 400 pulmonologists, cancer researchers, infections disease experts, and experts in critical care. TMPRSS2 may also play a role in African American disparities, as well as gender disparities, and this is also an active area of research for the Prostate Cancer Foundation.
About HITCH, a new clinical trial at the VA.
HITCH (Hormonal Intervention for the Treatment in Veterans with COVID-19 Requiring Hospitalization) is just one of the 10 new clinical trials developed with expertise from the PCF research enterprise to combat COVID-19. Led by PCF-funded researcher Dr. Matt Rettig at the West Los Angeles VA, HITCH will soon be recruiting eligible male vets who are over 18 and sick enough with COVID-19 to require hospitalization. To be eligible, patients must be hospitalized with mild-to-moderate disease but not be on a ventilator. The trial hopes to determine if we can stop patients from ever having to go on a ventilator. The trial will be recruiting nationally in the US.
HITCH is looking at whether degarelix, an FDA-approved drug for prostate cancer, can potentially help Veterans with COVID-19. Degarelix works by suppressing the body’s production of male hormones, which may then suppress COVID-19’s ability to gain entry to lung tissue.
Thanks to the PCF and VA partnership, which includes our $50 million network of 10 Centers of Excellence (COE), and the VA R&D office’s exceptionally rapid response to the pandemic – with a 7-days-a-week effort – the TMPRSS2 idea rapidly picked up speed… especially with PCF-VA centers in epicenters of the pandemic. The VA and the federal government worked at unprecedented speed to get this TMPRSS2 trial approved. The VA’s Chief of R&D, Rachel Ramoni, helped to fast-track the clinical trial protocol. Normally this process would take 10-12 months to get approved. Instead of the typical months-long clinical trial review process, it took only a matter of weeks to perform a multi-step rigorous review of this study.









