Informing care for intermediate-risk cancer, a new imaging tracer, and understanding the effects of PSA screening recommendations
Clinicians and researchers gathered last month at the American Society of Clinical Oncology Genitourinary Cancers Symposium (ASCO GU) — some in-person in San Francisco, some tuning in virtually. Hundreds of presentations covered advances in screening, diagnosis, treatment, monitoring, and survivorship in prostate and other GU cancers.
When making decisions about treating prostate cancer, it’s important to have as much information as possible. In Part 2 of ASCO GU highlights, we describe new research into two methods: 1) a closer look at biopsy tissue in patients with intermediate-risk prostate cancer, and 2) a type of highly-sensitive scan for prostate cancer metastases. Finally, one study examines the downstream effects of lack of information, as PSA screening declined in recent years.
Decipher gene test may help personalize care for patients with intermediate-risk prostate cancer
Prostate cancer is often assigned a risk group, ranging from very low to very high, based on factors such as PSA, Gleason score, DRE, and imaging. But there is variation within each risk group, and doctors and patients need additional information to identify which cancers may be more aggressive. Newer molecular biomarker tests – which look at biopsy tissue for many factors, such as expression of certain genes or proteins in the cancer – may provide extra guidance. One such test is called Decipher, but its use has yet not been well-defined in intermediate-risk prostate cancer.
PCF-funded investigator Dr. Daniel Spratt, Chair of Radiation Oncology at UH Seidman Cancer Center and Case Western Reserve University, presented findings based on a large trial of radiation therapy in patients with intermediate-risk prostate cancer. Biopsy samples from patients in the trial had been stored, some for many years. Spratt’s team ran the Decipher test on over 200 samples and looked at each patient’s clinical outcomes over an average of 13 years to see how they fared.
The team found that a higher Decipher score on the biopsy was linked to greater risk of poor outcomes such as disease progression, development of distant metastasis, and rising PSA. For example, patients with low Decipher scores had a 4% rate of distant metastasis by 10 years, compared to a 16% rate among patients with high Decipher scores.
What this means for patients: This study suggests that for patients with intermediate-risk prostate cancer, the Decipher test can be used to identify patients at greater risk of poor outcomes, based on their biopsy. Doctors can use this information to help personalize therapy.
SPOTLIGHT study results suggest that a new PSMA imaging agent is effective and safe
PSMA PET imaging was first FDA-approved in 2020. It can detect prostate cancer metastases earlier than with conventional imaging, when they are much smaller, which can help with treatment planning.
The SPOTLIGHT study tested a new PSMA-targeting imaging agent called 18F-rhPSMA-7.3 in about 400 men with suspected prostate cancer recurrence based on elevated PSA levels. Patients had imaging scans using 18F-rhPSMA-7.3 and the results were carefully read by a panel of experts. Overall, 18F-rhPSMA-7.3 was able to detect suspected lesions in 83% of patients across a wide range of PSA levels. At very low PSA levels (less than 0.5 ng/mL), the detection rate was 64%; this rate went up to 93% with a PSA between 1-2 ng/mL.
Because 18F-rhPSMA-7.3 is a new imaging method, next, the researchers confirmed these suspected lesions using established methods: biopsies and conventional imaging. 57% of patients had at least one confirmed lesion using both biopsy and conventional imaging as the “gold standard.” 81% of patients had at least one confirmed lesion using biopsy alone. This means that the trial achieved its threshold for correctly detecting prostate cancer lesions.
The agent was also safe, with no serious side effects related to it.
What this could mean for patients: Another option for highly sensitive prostate cancer imaging appears promising and is under study. This agent may have certain advantages vs currently available PSMA-targeting scans.
2012 recommendations against PSA screening linked to slower decline in prostate cancer death
Since its peak in 1993, the death rate from prostate cancer has dropped by over 50%. Imagine a “downhill” slope, heading towards zero. But if that trend line goes flat, it means that progress in the fight against the disease is stalled.
In 2012, the United States Preventive Services Task Force (USPSTF) recommended against routine PSA screening for prostate cancer, citing concerns that the risks of overdiagnosis and overtreatment outweighed the benefits. A team of researchers (including PCF-funded Young Investigator Dr. Sophia Kamran of Massachusetts General Hospital) examined how this recommendation may have affected death from prostate cancer between 1999-2019.
Using comprehensive data on U.S. prostate cancer deaths, PSA screening rates, and prostate cancer diagnoses, the team found that:
- Deaths from prostate cancer declined at a rate of (-)0.28 per 100,000/year from 1999 through 2012. However, from 2014-2019, the curve “flattened” – there was no change in the death rate.
- The effect was even more pronounced among certain groups of men. For example, among Black men from 1999 through 2012, the death rate was declining more sharply than for men overall, at the rate of (-)0.70 per 100,000/year (see Figure). From 2014-2019, the decline was significantly slower.
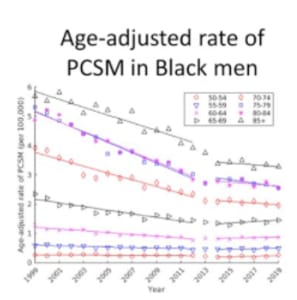
Also over this timeframe,
- PSA screening rates have dropped.
- Increasingly, men are found to have metastatic disease at the time of diagnosis.
- The death rate from cancer overall continued to decline, without this flattening.
Taken together, these results suggest that the 2012 recommendation against routine PSA screening contributed to the “leveling off” of the death rate from prostate cancer in recent years.
In 2018, the USPSTF recommended that the decision about PSA screening for men aged 55-69 be an individual one, together with their physician. Over time, this change may put us back on course for decreasing rates of death from prostate cancer, but it is too early to tell.
What this means for patients: Empower yourself with knowledge about your risk for prostate cancer. PCF encourages men to start talking with their doctor about prostate cancer screening at age 45, or age 40 if they have certain risk factors (Black race or a strong family history of prostate or other cancers). To help start the conversation, go to pcf.org/guides to download Things Every Man Should Know About Prostate Cancer.
Part 1: Highlights of ASCO GU 2022









